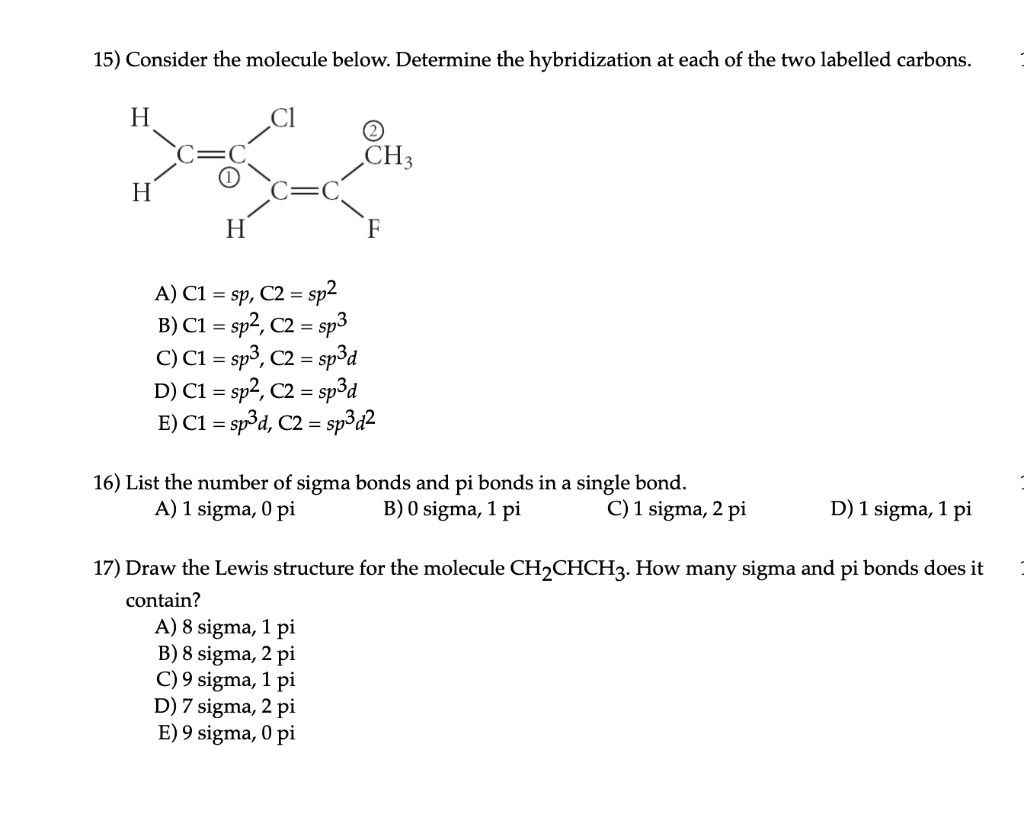
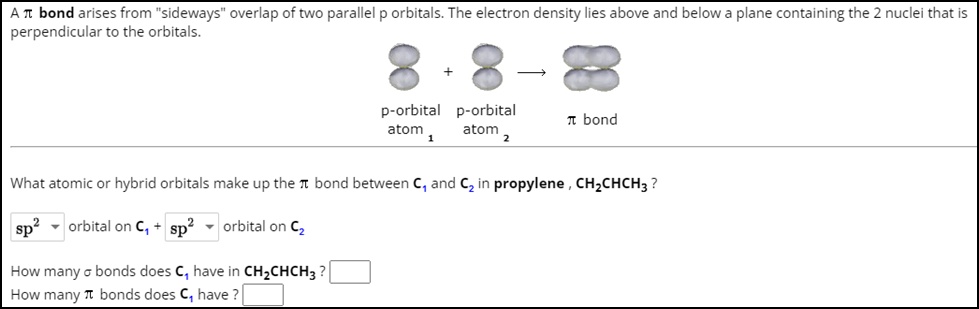
SOLVED: What atomic or hybrid orbitals make up the π bond between C1 and C2 in propylene, CH3(CHCH3)? (C1 is the first carbon in the formula as written: orbital on C1 orbital
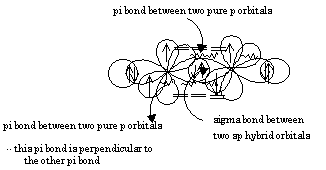
SOLVED: Texts: A bond arises from the "sideways" overlap of two parallel p orbitals. The electron density lies above and below a plane containing the two nuclei that is perpendicular to the

SOLVED: Text: Propane; CH3CH2CH3 and propene, CH3CH=CH2 are members of different homologous series. (1) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between carbon atoms. (ii) State

For each atom listed, identify the geometry as one of the following: tetrahedral, square planar, trigonal planar, trigonal pyramidal, or linear. | Homework.Study.com

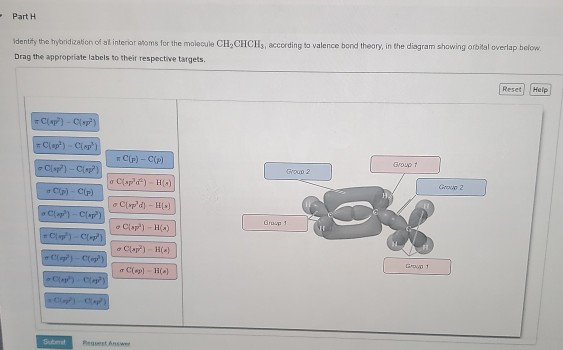
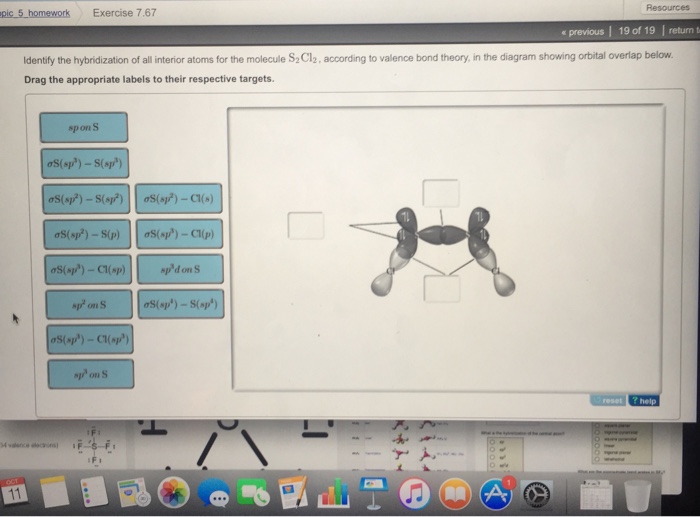
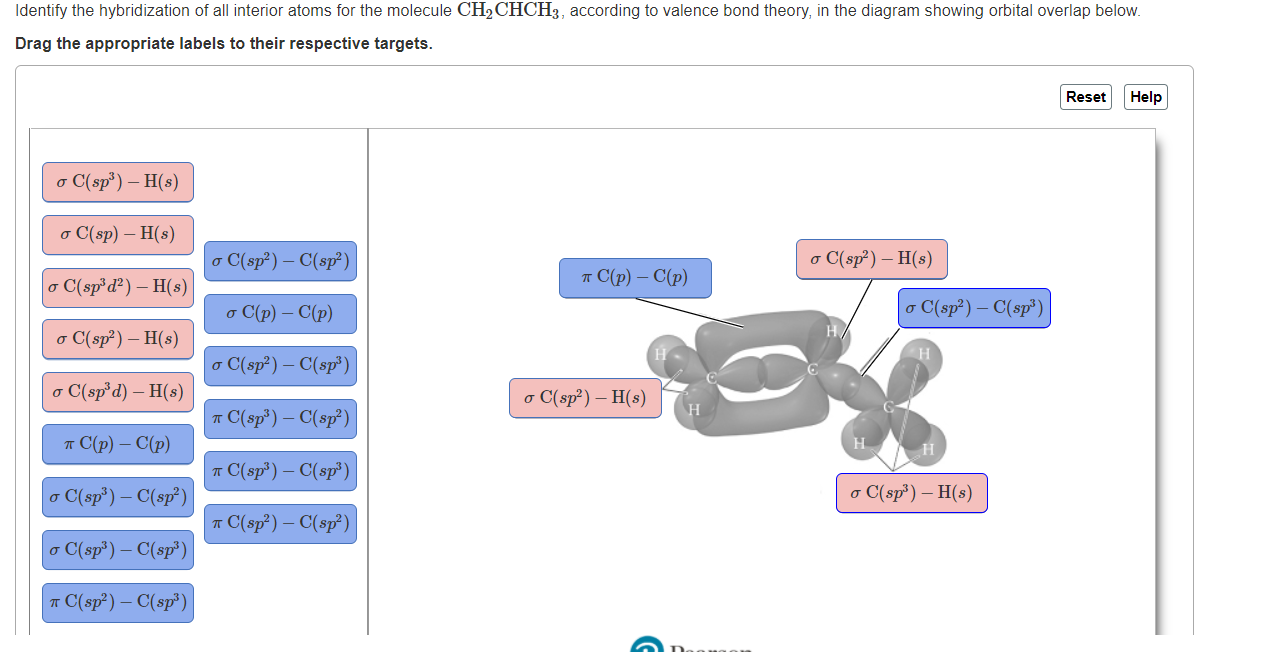
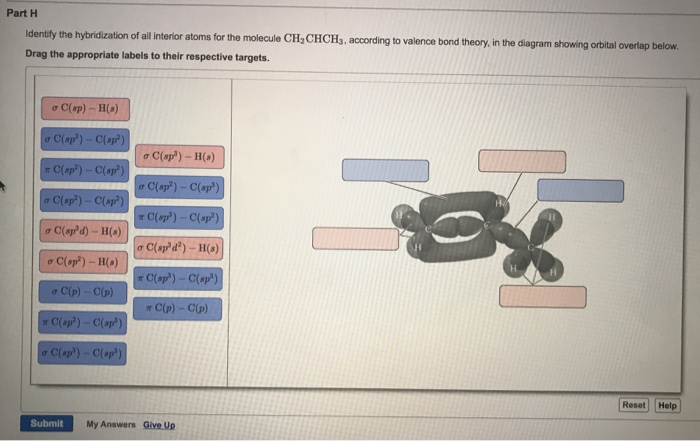
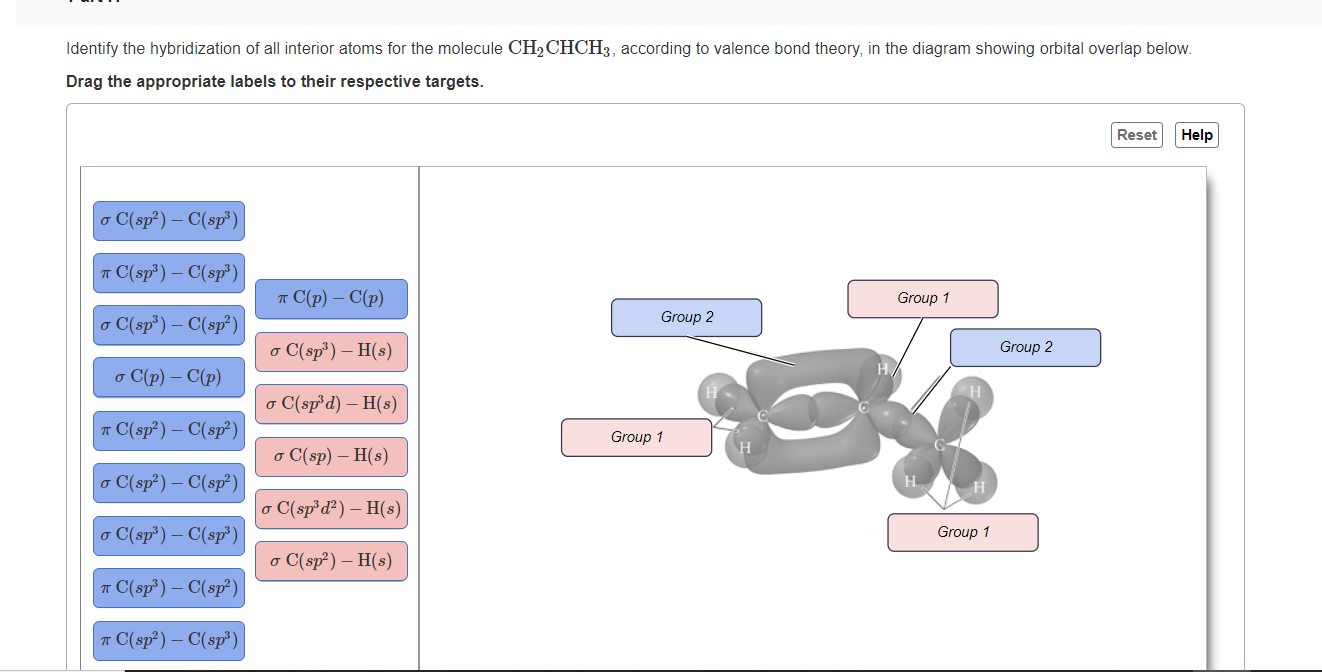
OneClass: Part H Identify the hybridization of all interior atoms for the molecule CH2 CHCHs, accordi...
Draw the Lewis structure for the molecule CH3CH2CCH. How many sigma and pi bonds does it contain? a) 11 sigma, 2 pi b) 9 sigma, 1 pi c) 8 sigma, 3 pi
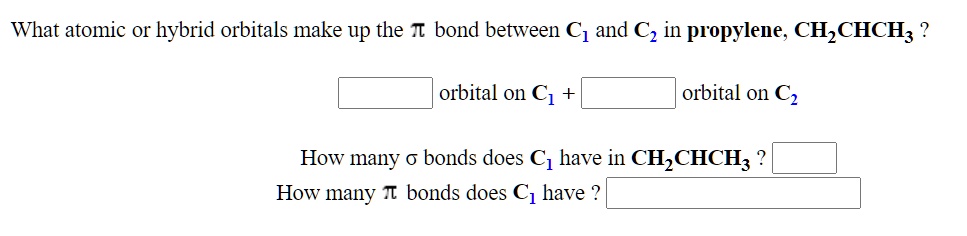
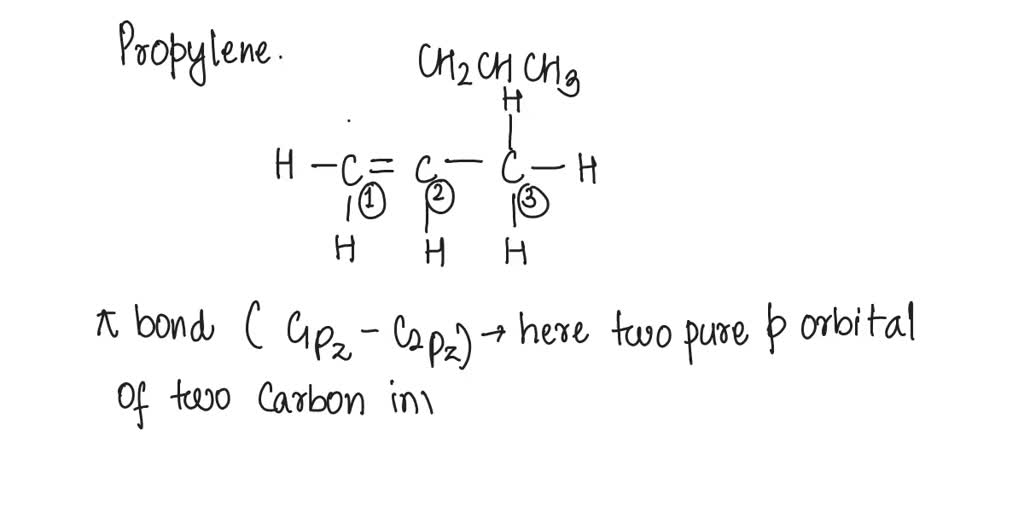
SOLVED: What atomic or hybrid orbitals make up the π bond between C1 and C2 in propylene, CH2CHCH3? π orbital on C1 π orbital on C2 How many bonds does C1 have

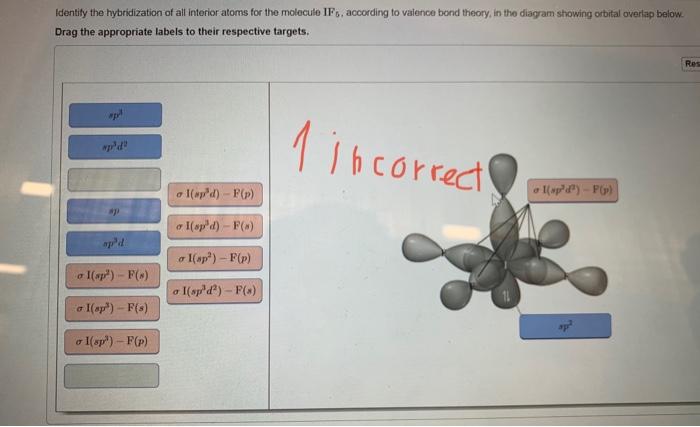
SOLVED: In valence bond theory, which orbital hybridization combination is found in O=C=O? A. 2s in carbon with a 2s in oxygen B. 2p in oxygen with a 2p in carbon C.

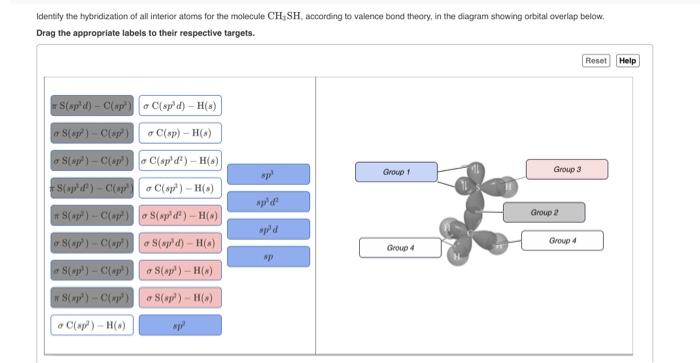
Draw all nonbonding hybrid orbitals and label the pi and sigma bonds for HCCCH_2OH. | Homework.Study.com
Write the type of hybridisation of each of the carbon atom in the following structures: (i) ch2=c=ch2 (ii) ch3 ch=ch ch3

Draw the lewis structure for the molecule ch2chch3. how many sigma and pi bonds does it 30) contain - brainly.com
University of Thi-Qar … College Of Science ……….. Department of Chemistry Organic Chemistry Second Stage Lecture 1 ( B