Hybridization and charge of Ni ions: a) Energy dependent hybridization... | Download Scientific Diagram
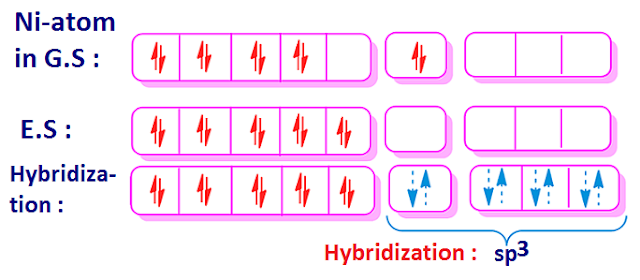
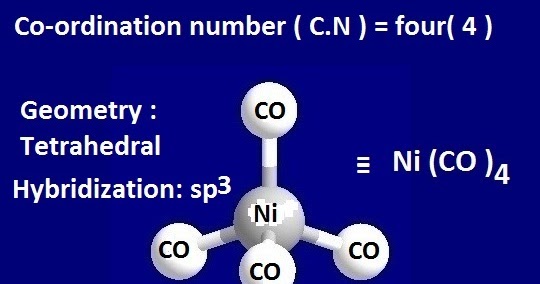
Why is Ni (CO) 4 tetrahedral and diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Write the Hybridization Type and Magnetic Behaviour of the Complex [Ni(Cn)4]2−. (Atomic Number of Ni = 28) - Chemistry | Shaalaa.com Write the Hybridization Type and Magnetic Behaviour of the Complex [Ni(Cn)4]2−. (Atomic Number of Ni = 28) - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:837e04eba2fc4496b5ad1a30a0ede108.png)
Write the Hybridization Type and Magnetic Behaviour of the Complex [Ni(Cn)4]2−. (Atomic Number of Ni = 28) - Chemistry | Shaalaa.com
![The hybridization of ${\\left[ {Nic{l_4}} \\right]^{2 - }}$ and ${\\left[ { Ni{{\\left( {CN} \\right)}_4}} \\right]^{2 - }}$ considering hybridization of the metal ion are respectively.(A) $s{p^3},ds{p^2}$(B) $ds{p^2},s{p^3}$(C) Both $s{p^3}$(D) Both ... The hybridization of ${\\left[ {Nic{l_4}} \\right]^{2 - }}$ and ${\\left[ { Ni{{\\left( {CN} \\right)}_4}} \\right]^{2 - }}$ considering hybridization of the metal ion are respectively.(A) $s{p^3},ds{p^2}$(B) $ds{p^2},s{p^3}$(C) Both $s{p^3}$(D) Both ...](https://www.vedantu.com/question-sets/292d2484-f6d0-482e-82eb-991506f4d8166691884101172465972.png)
The hybridization of ${\\left[ {Nic{l_4}} \\right]^{2 - }}$ and ${\\left[ { Ni{{\\left( {CN} \\right)}_4}} \\right]^{2 - }}$ considering hybridization of the metal ion are respectively.(A) $s{p^3},ds{p^2}$(B) $ds{p^2},s{p^3}$(C) Both $s{p^3}$(D) Both ...

Orbital polarization and pd hybridization Ni L3-edge X-ray absorption... | Download Scientific Diagram

Nd 5d–Ni 3d orbital hybridization and CDW in NdNiO2 and superconducting... | Download Scientific Diagram

Specifically, why is (Zn(CN)4)2- tetrahedral while (Ni(CN)4)2- is square planar, and why is (CuCl5)3- trigonal bipyramidal while (MnCl5)3- is square pyramidal? | Homework.Study.com
![Hybridization and geometry of [Ni(CN)_{4}]^{2-} are:sd^{3} and square planarsp^{2}d and tetrahedralsp^{3} and tetrahedraldsp^{2} and square planar Hybridization and geometry of [Ni(CN)_{4}]^{2-} are:sd^{3} and square planarsp^{2}d and tetrahedralsp^{3} and tetrahedraldsp^{2} and square planar](https://search-static.byjusweb.com/question-images/toppr_ext/questions/1453910_739662_ans_1b88a50e6b3142878d714df46d9472b7.png)
![Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube](https://i.ytimg.com/vi/5S_my6-2Vkc/maxresdefault.jpg)

![Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati](https://d10lpgp6xz60nq.cloudfront.net/physics_images/A2Z_CHM_XII_C09_E01_327_S01.png)
![Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube](https://i.ytimg.com/vi/r_C4yyTUSjM/mqdefault.jpg)
![What is the hybridization for [NiCN4]2 ? What is the hybridization for [NiCN4]2 ?](https://byjus-answer-creation.s3.amazonaws.com/uploads/2.14.jpg_img_upload_solution_2022-05-30%2005:07:29.453226.png)
![Solved] Hybridisation of [NiCl4]2- is ______. Solved] Hybridisation of [NiCl4]2- is ______.](https://storage.googleapis.com/tb-img/production/21/03/F1_Puja%20J_Anil_02.03.21_D9.png)
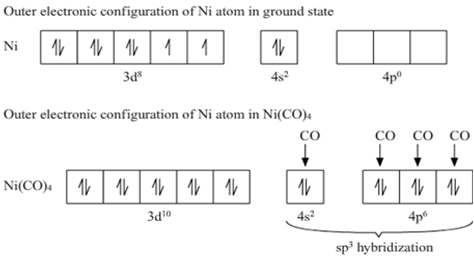
![Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:0c935226f0c2451f9878137028e5360f.png)
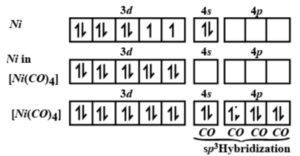
![Q.Find hybridisation and geometry of [Ni(CO)4] - YouTube Q.Find hybridisation and geometry of [Ni(CO)4] - YouTube](https://i.ytimg.com/vi/5rlnQONyoeU/hqdefault.jpg)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
![Ni(NH3)6]+3 hybridisation structure - YouTube Ni(NH3)6]+3 hybridisation structure - YouTube](https://i.ytimg.com/vi/iZYGIu-3Fls/maxresdefault.jpg)