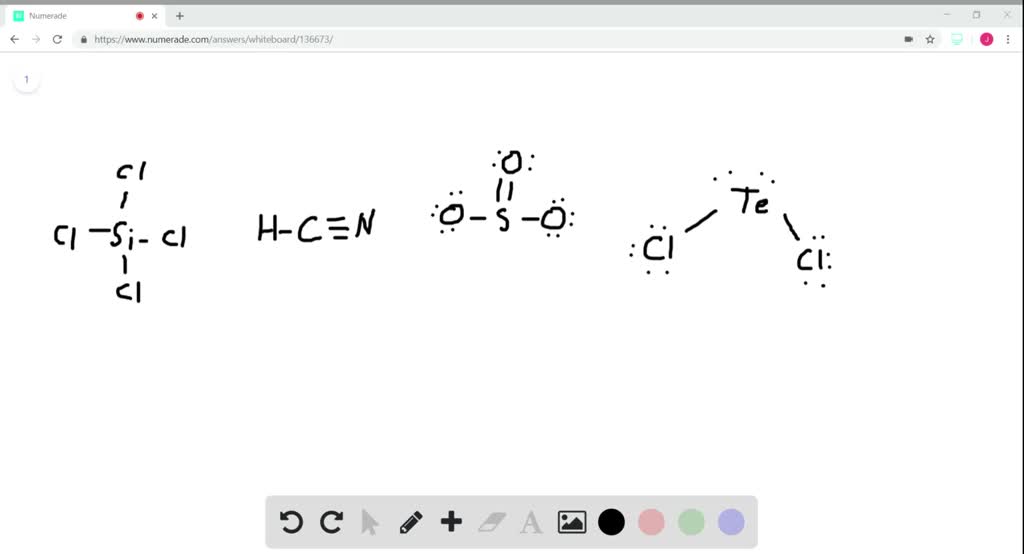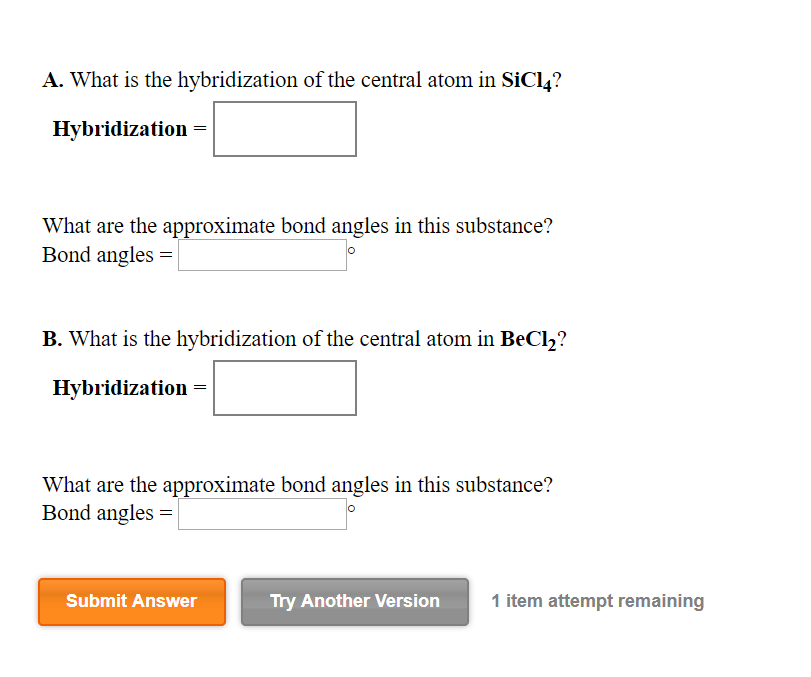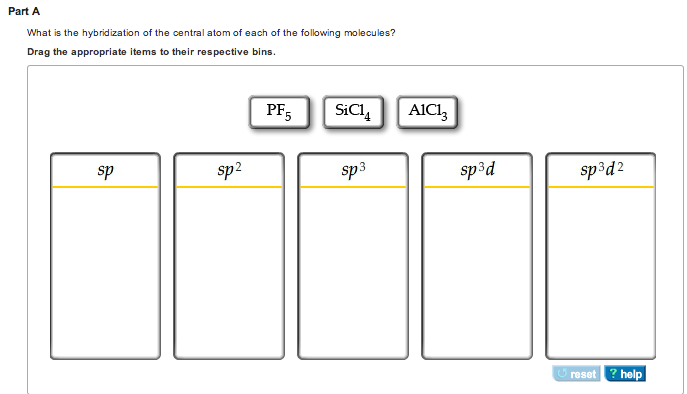
What is the hybridization of the central atom of each of the following molecules? - Home Work Help - Learn CBSE Forum
Q. Which of the following pair has same hybridisation. 1] BF3 NF3 2] SF4 SiCl4 3] ClO4 ClO2 4] CO2 SiO2
Hybridization is a phenomenon that takes place in an atom before chemical bonding. How is hybridization responsible for the observed structure of SiCl4? - Quora
In case of hybridization in chemical bonding is only the central atom hybridized or atoms also except central atoms hybridize to form bonds? What is the exact concept of hybridization? - Quora
Q. Which of the following pair has same hybridisation. 1] BF3 NF3 2] SF4 SiCl4 3] ClO4 ClO2 4] CO2 SiO2
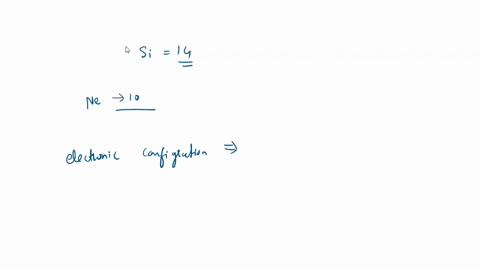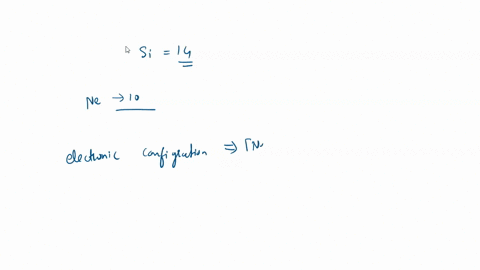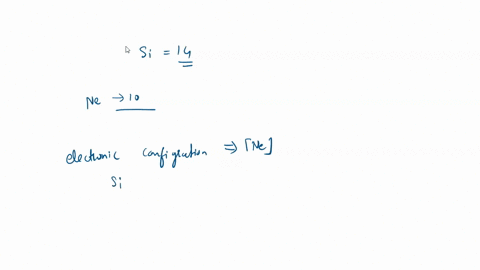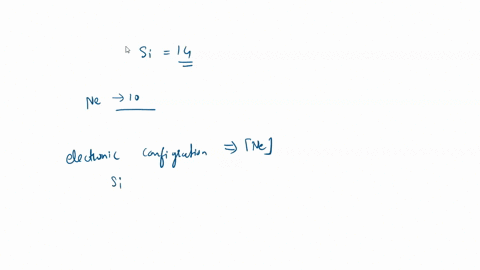
SOLVED: For the tetrahedral molecule, silicon tetrachloride, SiCl4, write the condensed electron configuration of a lone silicon atom, predict the hybridization in the molecule, and write an electron configuration for the hybridized orbitals of the central silicon ...
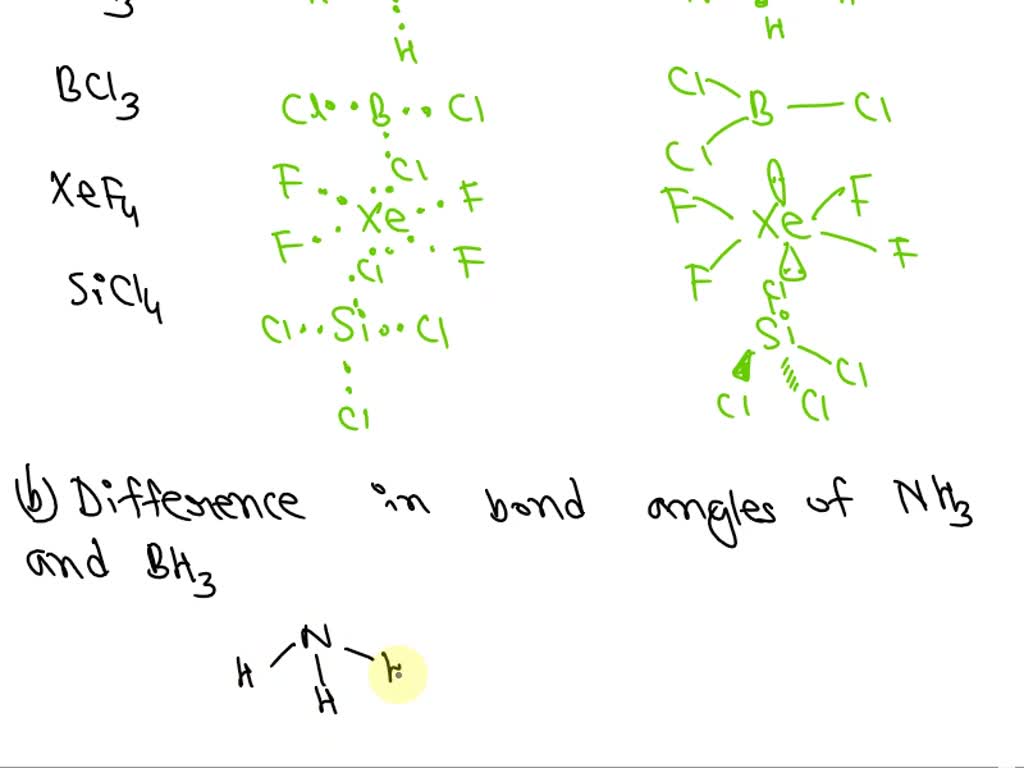
SOLVED: a) Draw the Lewis structures and molecular shapes of NH3, BH3, PCl5, XeF4 and SiCl4, and indicate the hybridization types and geometric shapes. b) Compare the bond angles in NH3 and
Silicon tetrachloride SiCl4: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure –
We all know that CCl4 and SiCl4 both compound remains in Tetrahedral structure , with a hybridization of sp³ in the central atom. Now , Si is a much larger atom than

BCU-NEP-IV SEM-DSC-04-Chemical bonding-Valence Bond Theory(VBT)Sp3 Hybridization-SiCl4 as an Example - YouTube